Question:
Why does boron trifluoride behave as a Lewis acid?
Solution:
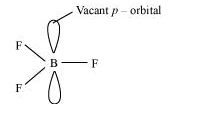
The electric configuration of boron is ns2 np1. It has three electrons in its valence shell. Thus, it can form only three covalent bonds. This means that there are only six electrons around boron and its octet remains incomplete. When one atom of boron combines with three fluorine atoms, its octet remains incomplete. Hence, boron trifluoride remains electron-deficient and acts as a Lewis acid.