Why are detergents better cleansing agents than soaps ? Explain.
A substance capable of removing grease and dirt from any fabric or body is called detergent. The detergents are of two types Le. soapy and non-
soapy detergents. The soapy detergents are soaps whereas the non-soapy detergents are synthetic detergents or simply detergents. Although
both are cleansing agents, they differ in chemical composition. In the present chapter, we shall briefly discuss the composition and cleansing
action of soaps and synthetic detergents.
Soaps are the sodium and potassium salts of long chain fatty acids with general formula RCOONa or RCOOK. The acids present have the
formula RCOOH where R may have following values.

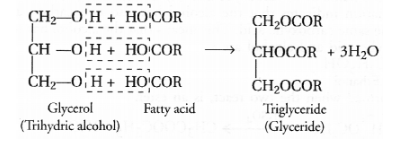
These fatty acids exist as triesters of glycerol which is a trihydric alcohol. The triesters are also called triglycerides or simply glycerides and are the
constituents of edible oils and fats. These are of animal and vegetable origin e.g. castor oil, linseed oil or soyabean oil. Chemically the
triglycerides are formed as a result of esterification reaction.