Question:
Which one of the following graphs is not correct for ideal gas?
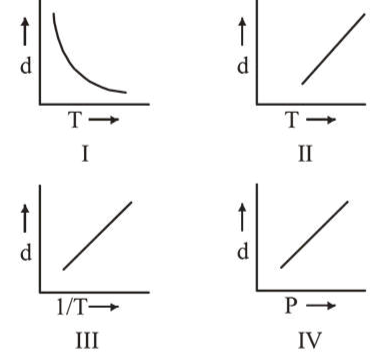
$\mathrm{d}=$ Density, $\mathrm{P}=$ Pressure, $\mathrm{T}=$ Temperature
Correct Option: , 2
Solution:
For ideal gas
$P V=n R T$
$P V=\frac{m}{M} R T \quad\left(\because n=\frac{m}{M}\right)$
$P M=\frac{m}{V} R T ; \quad P M=d R T ; \quad d=\left[\frac{P M}{R}\right] \frac{1}{T}$
$\Rightarrow d \propto \frac{1}{T} ; \quad d \propto P$
So, graph between $d \mathrm{Vs} T$ is not straight line.
