Question:
Which one is the correct option for the two different thermodynamic processes?
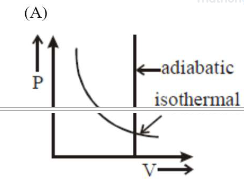
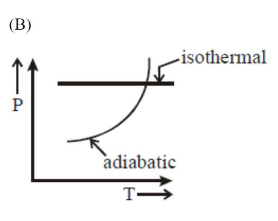
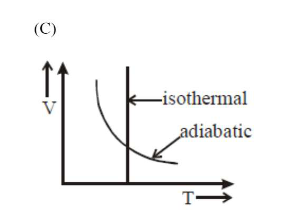
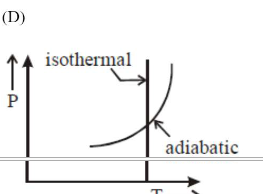
Correct Option: , 2
Solution:
(2)
Option (a) is wrong; since in adiabatic
process $V \neq$ constant.
Option (b) is wrong, since in isothermal process
$T=$ constant
Option (c) $\backslash \&$
(d) matches isothermes $\backslash \&$
adiabatic formula:
constant $\backslash \& \frac{\mathrm{T}^{\gamma}}{\mathrm{p}^{\gamma-1}}=$ constant
