Question: Which of the following is least basic ?
$\left(\mathrm{CH}_{3} \mathrm{CO}\right) \ddot{\mathrm{N}} \mathrm{HC}_{2} \mathrm{H}_{5}$
$\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \ddot{\mathrm{N}}$
$\left(\mathrm{CH}_{3} \mathrm{CO}\right)_{2} \ddot{\mathrm{N}} \mathrm{H}$
$\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{2} \ddot{\mathrm{N}} \mathrm{H}$
Correct Option: , 3
Solution:
For the given compounds :

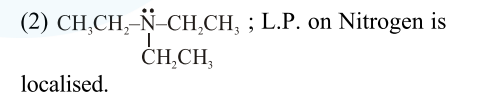


(Hence least basic)
(4) $\mathrm{CH}_{3}-\mathrm{CH}_{2}-\ddot{\mathrm{NH}}-\mathrm{CH}_{2}-\mathrm{CH}_{3} ;$ L.P. on Nitrogen is localised.