Question:
Which of the following has the shortest $\mathrm{C}-\mathrm{Cl}$ bond?
Correct Option: , 4
Solution:
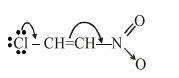
Due to $-\mathrm{M}$ effect of $-\mathrm{NO}_{2}$ and $+\mathrm{M}$ effect of $\mathrm{Cl}$ more D.B. character between $\mathrm{C}-\mathrm{Cl}$ bond. So shortest bond length.
