Question:
Which of the following has maximum number of atoms?
(a) $18 \mathrm{~g}$ of $\mathrm{H}_{2} \mathrm{O}$
(b) $18 \mathrm{~g}$ of $\mathrm{O}_{2}$
(c) $18 \mathrm{~g}$ of $\mathrm{CO}_{2}$
(d) $18 \mathrm{~g}$ of $\mathrm{CH}_{4}$
Solution:
(d)
No. of atoms =
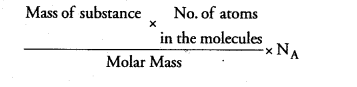
(a) $\frac{(18 \mathrm{~g}) \times 3}{(18 \mathrm{~g})} \times \mathrm{N}_{\mathrm{A}}=3 \mathrm{~N}_{\mathrm{A}}$
(b) $\frac{(18 \mathrm{~g}) \times 2}{(32 \mathrm{~g})} \times \mathrm{N}_{\mathrm{A}}=1.23 \mathrm{~N}_{\mathrm{A}}$
(c) $\frac{(18 \mathrm{~g}) \times 3}{(44 \mathrm{~g})} \times \mathrm{N}_{\mathrm{A}}=1.23 \mathrm{~N}_{\mathrm{A}}$
(d) $\frac{(18 \mathrm{~g}) \times 5}{(16 \mathrm{~g})} \times \mathrm{N}_{\mathrm{A}}=5.63 \mathrm{~N}_{\mathrm{A}}$
