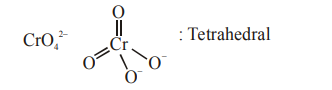Question:
Which of the following are isostructural pairs ?
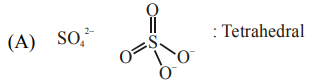
A. $\mathrm{SO}_{4}^{2-}$ and $\mathrm{CrO}_{4}^{2-}$
B. $\mathrm{SiCl}_{4}$ and $\mathrm{TiCl}_{4}$
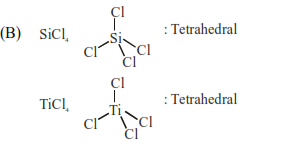
C. $\mathrm{NH}_{3}$ and $\mathrm{NO}_{3}^{-}$
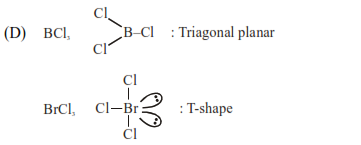
D. $\mathrm{BCl}_{3}$ and $\mathrm{BrCl}_{3}$
$\mathrm{BCl}_{3}$ and $\mathrm{BrCl}_{3}$
Correct Option: , 2
Solution:
Isostructural means same structure