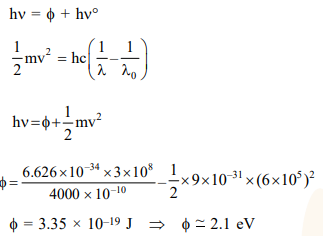Question:
What is the work function of the metal if the light of wavelength $4000 \AA$ generates photoelectrons of velocity $6 \times 10^{5} \mathrm{~ms}^{-1}$ form it ?
(Mass of electron $=9 \times 10^{-31} \mathrm{~kg}$
Velocity of light $=3 \times 10^{8} \mathrm{~ms}^{-1}$
Planck's constant $=6.626 \times 10^{-34} \mathrm{Js}$
Charge of electron $=1.6 \times 10^{-19} \mathrm{JeV}^{-1}$ )
Correct Option: , 3
Solution: