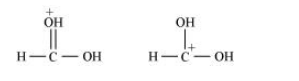What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors?
(a)
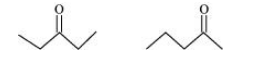
(b)

(c)
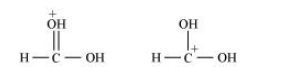
(a) Compounds having the same molecular formula but with different structures are called structural isomers. The given compounds have the same molecular formula but they differ in the position of the functional group (ketone group).
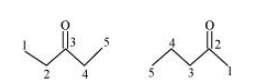
In structure I, ketone group is at the C-3 of the parent chain (hexane chain) and in structure II, ketone group is at the C-2 of the parent chain (hexane chain). Hence, the given pair represents structural isomers.
(b) Compounds having the same molecular formula, the same constitution, and the sequence of covalent bonds, but with different relative position of their atoms in space are called geometrical isomers.

In structures I and II, the relative position of Deuterium (D) and hydrogen (H) in space are different. Hence, the given pairs represent geometrical isomers.
(c) The given structures are canonical structures or contributing structures. They are hypothetical and individually do not represent any real molecule. Hence, the given pair represents resonance structures, called resonance isomers.