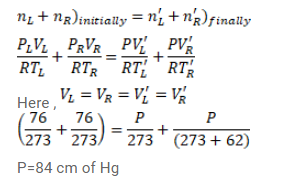Question:
Use $R=8.3 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$ wherever required.
Two glass bulbs of equal volume are connected by a narrow tube and are filled with a gas at $0^{\circ} \mathrm{C}$ at a pressure of $76 \mathrm{~cm}$ of mercury. One of the bulbs is then placed in a water bath maintained at $62^{\circ} \mathrm{C} .$ What is the new value of the pressure inside the bulbs? The volume of the connecting tube is negligible.
Solution: