Question:
Use $R=8.3 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$ wherever required.
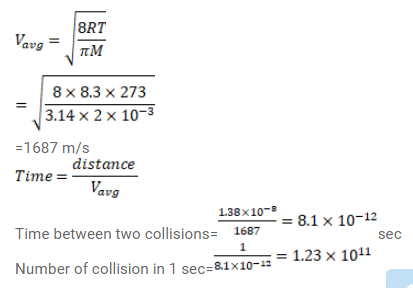
Estimate the number of collisions per second suffered by a molecule in a sample of hydrogen at STP. The mean free path (average distance covered by a molecule between successive collisions) $=1.38 \times 10^{-5}$ $\mathrm{cm}$.
Solution: