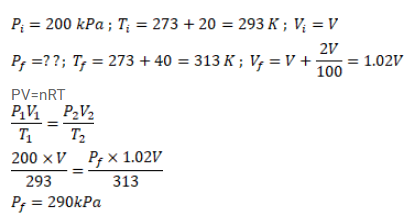Question:
Use $R=8.3 \mathrm{~J} / \mathrm{mol}-\mathrm{K}$ wherever required.
Air is pumped into an automobile tyre's tube upto a pressure of $200 \mathrm{kPa}$ in the morning when the air temperature is $20^{\circ} \mathrm{C}$. During the day the temperature rises to $40^{\circ} \mathrm{C}$ and the tube expands by $2 \%$. Calculate the pressure of the air in the tube at this temperature.
Solution: