Question:
thermodynamic process is shown below on a P-V diagram for one mole of an ideal gas. If $V_{2}=2 V_{1}$ then the ratio of temperature $T_{2} / T_{1}$ is:
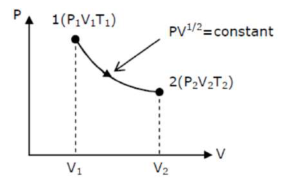
Correct Option: , 4
Solution:
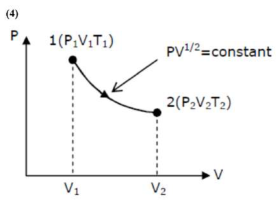
From $\mathrm{p}-\mathrm{v}$ diagram,
Given $\mathrm{Pv}^{1 / 2}=$ constant $\ldots$ (i)
We know that
$\mathrm{PV}=\mathrm{nRT}$
$P \propto\left(\frac{T}{v}\right)$
Put in equation (i)
$\left(\frac{T}{v}\right)(v)^{1 / 2}=$ constant
$T \propto v^{1 / 2}$
$\frac{T_{2}}{T_{1}}=\sqrt{\frac{V_{2}}{V_{1}}}$
$\frac{T_{2}}{T_{1}}=\sqrt{\frac{2 \sigma_{1}}{V_{2}}}$
$\frac{T_{2}}{T_{1}}=\sqrt{2}$
