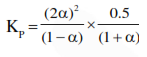Question:
The value of $\mathrm{Kp}$ for the equilibrium reaction $\mathrm{N}_{2} \mathrm{O}_{4}(\mathrm{~g}) \rightleftharpoons 2 \mathrm{NO}_{2}(\mathrm{~g})$ is 2 . The percentage dissociation of $\mathrm{N}_{2} \mathrm{O}_{4}(\mathrm{~g})$ at a pressure of $0.5 \mathrm{~atm}$ is
Correct Option: 1
Solution: