Question:
The structure of triphenylmethylcation is given below. This is very stable and some of its salts can be stored for months. Explain the cause of the high stability of this cation.
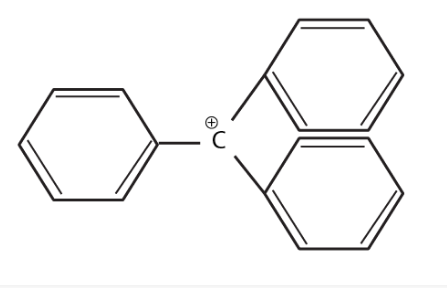
Solution:
Triphenylcarbocation is tertiary carbocation and the positive charge is on the carbon atom which is stabilized by the three phenyl group by resonance. Due to the
resonance the stability increases.
