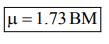Question:
The spin-only magnetic moment value for the complex $\left[\mathrm{Co}(\mathrm{CN})_{6}\right]^{4-}$ is BM.
[At. no. of $\mathrm{Co}=27]$
Solution:
$\left[\mathrm{Co}(\mathrm{CN})_{6}\right]^{4-}$
$x+6 \times(-1)=-4$
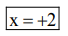
$\mathrm{Co}^{2+}:[\mathrm{Ar}] 3 \mathrm{~d}^{7}$
and $\mathrm{CN}^{-}$is a strong field ligand which can pair electron of central atom.
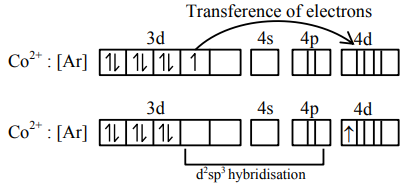
It has one unpaired electron (n) in 4d-subshell.
So spin only magnetic moment $(\mu)=$
$\sqrt{\mathrm{n}(\mathrm{n}+2)}$ B.M
where $\mathrm{n}=$ number of unpaired electrons.
$\mu=\sqrt{3} \quad$ B.M