Question:
The spin only magnetic moment of a divalent ion in aqueous solution (atomic number 29) is__________ BM.
Solution:
$\mathrm{Z}=29(\mathrm{Cu})$
$\mathrm{Cu}^{2+}$ form $\left[\mathrm{Cu}\left(\mathrm{H}_{2} \mathrm{O}\right)_{4}\right]^{2+}$ complex ion with $\mathrm{H}_{2} \mathrm{O}$.$\left[\mathrm{Cu}\left(\mathrm{H}_{2} \mathrm{O}\right)_{4}\right]^{2+} \Rightarrow \mathrm{Cu}^{2+}[\mathrm{Ar}] 3 \mathrm{~d}^{9}, \mathrm{H}_{2} \mathrm{O} \rightarrow$ WFL
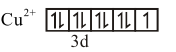
number of unpaired $\mathrm{e}^{-}=1$
$\mu=\sqrt{1(1+2)}$ B.M.
$\mu=\sqrt{3} \Rightarrow 1.73$ B.M. $\Rightarrow$ round off ans. $\Rightarrow 2$
