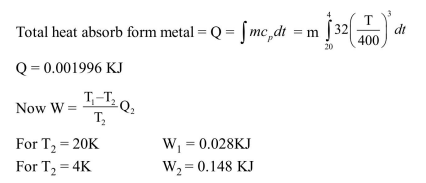Question:
The specific heat capacity of a metal at low temperautre $(T)$ is given as $C_{p}\left(k J J k^{-1} k g^{-1}\right)=32\left(\frac{T}{400}\right)^{3}$
A 100 gram vessel of this metal is to be cooled from $20^{\circ} \mathrm{K}$ to $4^{\circ} \mathrm{K}$ by a special refrigerator operating at room temperature $\left(27^{\circ} \mathrm{C}\right)$. The amount of work required to cool the vessel is:-
Correct Option: , 3
Solution: