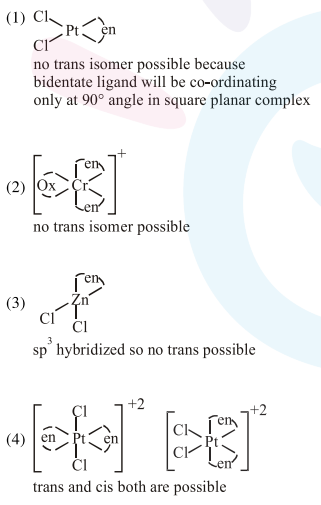
Question: The species that can have a trans-isomer is :
(en $=$ ethane-1, 2 -diamine, ox $=$ oxalate)
$\left[\mathrm{Pt}(\mathrm{en}) \mathrm{Cl}_{2}\right]$
$\left[\mathrm{Cr}(\mathrm{en})_{2}(\mathrm{ox})\right]^{+}$
$\left[\mathrm{Zn}(\mathrm{en}) \mathrm{Cl}_{2}\right]$
$\left[\mathrm{Pt}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{2+}$
Correct Option: , 4
Solution: