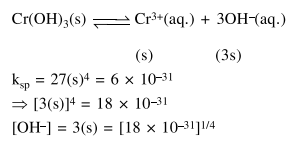Question: The solubility product of $\mathrm{Cr}(\mathrm{OH})_{3}$ at $298 \mathrm{~K}$ is $6.0 \times 10^{-31}$. The concentration of hydroxide ions in a saturated solution of $\mathrm{Cr}(\mathrm{OH})_{3}$ will be :
$\left(18 \times 10^{-31}\right)^{1 / 4}$
$\left(2.22 \times 10^{-31}\right)^{1 / 4}$
$\left(4.86 \times 10^{-29}\right)^{1 / 4}$
$\left(18 \times 10^{-31}\right)^{1 / 2}$
Correct Option: 1
Solution: