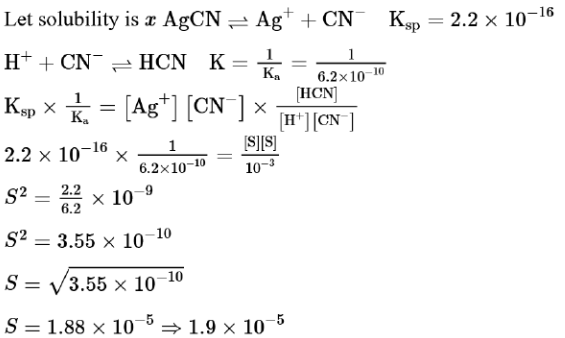
Question:
The solubility of $\mathrm{AgCN}$ in a buffer solution of $\mathrm{pH}=3$ is $\mathrm{x} .$ The value of $\mathrm{X}$ is:
[Assume: No cyano complex is formed; $\mathrm{K}_{\mathrm{sp}}(\mathrm{AgCN})=2.2 \times 10^{-16}$ and $\mathrm{K}_{\mathrm{a}}(\mathrm{HCN})=6.2 \times 10^{-10}$ ]
Correct Option: , 4
Solution: