Question:
The solubility of $\mathrm{Ca}(\mathrm{OH})_{2}$ in water is :
[Given : The solubility product of $\mathrm{Ca}(\mathrm{OH})_{2}$ in water $=5.5 \times 10^{-6}$ ]
Correct Option: , 3
Solution:
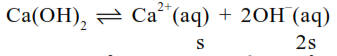
$\mathrm{k}_{\mathrm{sp}}=\mathrm{s}(2 \mathrm{~s})^{2} \Rightarrow 5.5 \times 10^{-6}=4 \mathrm{~S}^{3}$
$\Rightarrow \mathrm{s}=\left(\frac{5.5}{4}\right)^{\frac{1}{3}} \times 10^{-2}=1.11 \times 10^{-2}$
