Question:
The secondary valency and the number of hydrogen bonded water molecule(s) in $\mathrm{CuSO}_{4} \cdot 5 \mathrm{H}_{2} \mathrm{O}$, respectively, are
Correct Option: , 2
Solution:
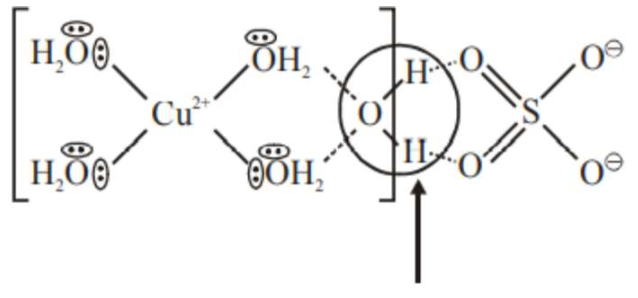
Hydrogen bonded water molecule $=1$ Secondary valency $=4$
