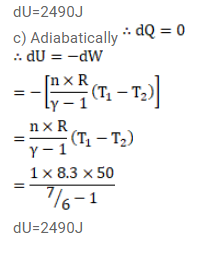Question:
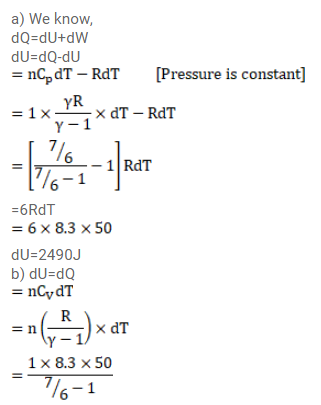
The ratio of the molar heat capacities of an ideal gas is $C_{p} / C_{v}=7 / 6$. Calculate the change in internal energy of $1.0$ mol of the gas when its temperature is raised by $50 \mathrm{~K}$
(a) keeping the pressure constant,
(b) keeping the volume constant and
(c) adiabatically.
Solution: