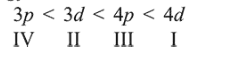Question:
The quantum number of four electrons are given below :
I. $\quad n=4, l=2, m_{l}=-2, m_{s}=-1 / 2$
II. $n=3, l=2, m_{l}=1, m_{s}=+1 / 2$
III. $n=4, l=1, m_{l}=0, m_{s}=+1 / 2$
IV. $n=3, l=1, m_{l}=1, m_{\mathrm{s}}=-1 / 2$
The correct order of their increasing energies will be :
Correct Option: , 3
Solution:
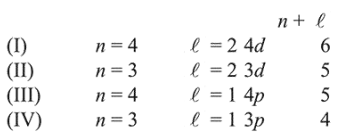
The energy of an atomic orbital increases with increasing $n+\ell$. For identical values of $n+\ell$, energy increases with increasing $\mathrm{n}$. Therefore the correct order of energy is: