Question:
The presence of soluble fluoride ion upto $1 \mathrm{ppm}$ concentration in drinking water, is :
Correct Option: , 4
Solution:
Fluoride $\left(\mathrm{F}^{-}\right)$ion conc. upto $1 \mathrm{ppm}$ makes the enamel of teeth much harder by converting
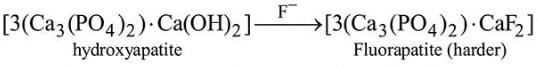
But above 2 ppm cause brown motting of teeth.
