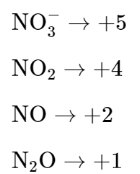Question: The oxidation states of nitrogen in $\mathrm{NO}, \mathrm{NO}_{2}$,
$\mathrm{N}_{2} \mathrm{O}$ and $\mathrm{NO}_{3}^{-}$are in the order of:
$\mathrm{NO}_{3}^{-}>\mathrm{NO}_{2}>\mathrm{NO}>\mathrm{N}_{2} \mathrm{O}$
$\mathrm{NO}_{2}>\mathrm{NO}_{3}^{-}>\mathrm{NO}>\mathrm{N}_{2} \mathrm{O}$
$\mathrm{N}_{2} \mathrm{O}>\mathrm{NO}_{2}>\mathrm{NO}>\mathrm{NO}_{3}^{-}$
$\mathrm{NO}>\mathrm{NO}_{2}>\mathrm{N}_{2} \mathrm{O}>\mathrm{NO}_{3}^{-}$
Correct Option: 1
Solution:
The oxidation states of Nitrogen in following molecules are as follows