Question:
The number of stereoisomers possible for $\left[\mathrm{Co}(\mathrm{ox})_{2}(\mathrm{Br})\left(\mathrm{NH}_{3}\right)\right]^{2-}$ is________ . $[\mathrm{ox}=$ oxalate $]$
Solution:
Total number of stereoisomers in $\left[\mathrm{Co}(\mathrm{ox})_{2} \mathrm{Br}\left(\mathrm{NH}_{3}\right)\right]^{2 \mathrm{O}}$ i.e. $\simeq\left[\mathrm{M}(\mathrm{AA})_{2} \mathrm{ab}\right]^{2-}$
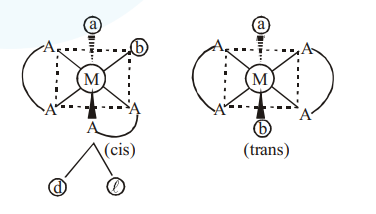
$\rightarrow$ cis is optically active isomers and trans is optically inactive isomer
$\rightarrow$ Hence total isomers is $=3$
