The number of f electrons in the ground state electronic configuration of Np (Z = 93) is ________. (Nearest integer)
Question:
The number of f electrons in the ground state electronic configuration of Np (Z = 93) is ________. (Nearest integer)
Solution:
$\mathrm{Np}=1 \mathrm{~s}^{2} 2 \mathrm{~s}^{2} 2 \mathrm{p}^{6} 3 \mathrm{~s}^{2} 3 \mathrm{p}^{6} 4 \mathrm{~s}^{2} 3 \mathrm{~d}^{10} 4 \mathrm{p}^{6} 5 \mathrm{~s}^{2} 4 \mathrm{~d}^{10} 5 \mathrm{p}^{6} 6 \mathrm{~s}^{2}$
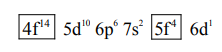
Total no. of 'f' electron $=14 \mathrm{e}^{-}+4 \mathrm{e}^{-}=18$
