Question:
The mole fraction of glucose $\left(\mathrm{C}_{6} \mathrm{H}_{12} \mathrm{O}_{6}\right)$ in an aqueous binary solution is $0.1$. The mass percentage of water in it, to the nearest integer, is ___________ .
Solution:
(47)
Let total mole of solution $=1$
So, mole of glucose $=0.1$
Mole of $\mathrm{H}_{2} \mathrm{O}=0.9$
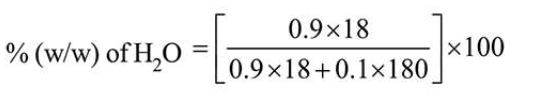
$=47.368=47.37$
