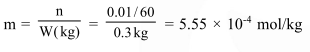Question: The molality of a urea solution in which $0.0100 \mathrm{~g}$ of urea, $\left[\left(\mathrm{NH}_{2}\right)_{2} \mathrm{CO}\right]$ is added to $0.3000 \mathrm{dm}^{3}$ of water at STP is :-
0.555 m
$5.55 \times 10^{-4} \mathrm{~m}$
33.3 m
$3.33 \times 10^{-2} \mathrm{~m}$
Correct Option: , 2
Solution: