The ionization enthalpy of $\mathrm{Na}^{+}$formation from $\mathrm{Na}_{(\mathrm{g})}$ is $495.8 \mathrm{~kJ} \mathrm{~mol}^{-1}$, while the electron gain enthalpy of $\mathrm{Br}$ is $-325.0 \mathrm{~kJ} \mathrm{~mol}^{-1}$. Given the lattice enthalpy of $\mathrm{NaBr}$ is $-728.4 \mathrm{~kJ} \mathrm{~mol}^{-1}$. The energy for the formation of $\mathrm{NaBr}$ ionic solid is $(-)$ ___________$\times 10^{-1} \mathrm{~kJ} \mathrm{~mol}^{-1}$.
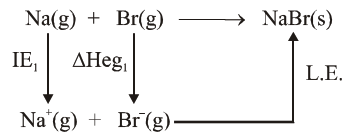
$\Delta \mathrm{H}_{\text {formation }}=\mathrm{IE}_{1}+\Delta \mathrm{Heg}_{1}+\mathrm{LE}$
$=495.8+(-325)+(-728.4)$
$=-557.6$
$=-5576 \times 10^{-1} \mathrm{KJ} / \mathrm{mol}$
Note: The above calculation is not for $\Delta \mathrm{H}_{\text {formation }}$ but for $\Delta \mathrm{H}_{\text {Reaction. }}$
But on the basis of given data it is the best ans.
