Question:
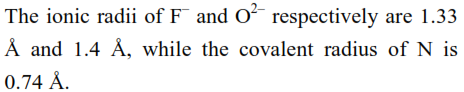
The correct statement for the ionic radius of $\mathrm{N}^{3-}$ from the following is :
Correct Option: , 2
Solution:
$\mathrm{F}^{-}, \mathrm{O}^{2-}$ and $\mathrm{N}^{3-}$ all are isoelectronic species in which $\mathrm{N}^{3-}$ have least number of protons due to which it's size increases as least nuclear attraction is experienced by the outer shell electrons.
Size order $\quad \mathrm{N}^{3-}>\mathrm{O}^{2-}>\mathrm{F}^{-}$
