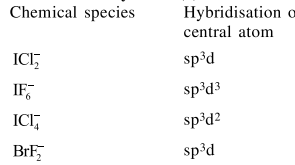
Question: The ion that has $s p^{3} \mathrm{~d}^{2}$ hybridization for the central atom, is :
$\left[\mathrm{ICI}_{2}\right]^{-}$
$\left[\mathrm{IF}_{6}\right]^{-}$
$\left[\mathrm{ICI}_{4}\right]^{-}$
$\left[\mathrm{BrF}_{2}\right]^{-}$
Correct Option: , 3
Solution: