Question:
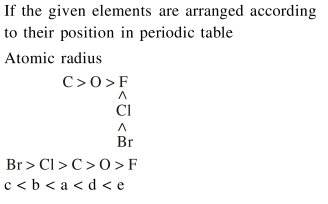
The increasing order of the atomic radii of the following elements is :-
(a) $\mathrm{C}$
(b) $\mathrm{O}$
(c) $\mathrm{F}$
(d) $\mathrm{Cl}$
(e) $\mathrm{Br}$
Correct Option: , 4
Solution:
Click here to get exam-ready with eSaral
For making your preparation journey smoother of JEE, NEET and Class 8 to 10, grab our app now.
