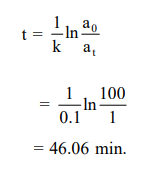Question:
The half life period of a first order chemical reaction is 6.93 minutes. The time required for the completion of 99% of the chemical reaction will be (log 2 = 0.301) :-
Correct Option: , 2
Solution:
$\mathrm{k}=\frac{0.693}{\mathrm{t}_{1 / 2}}=\frac{0.693}{6.93}=0.1 \mathrm{~min}^{-1}$