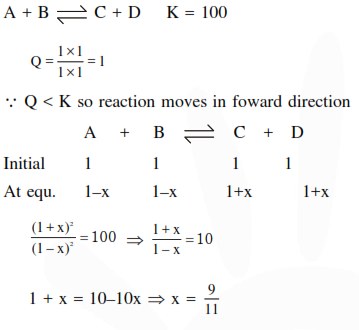
Question:
The equilibrium constants at $298 \mathrm{~K}$ for a reaction $\mathrm{A}+\mathrm{B} \rightleftharpoons \mathrm{C}+\mathrm{D}$ is 100 . If the initial concentration of all the four species were $1 \mathrm{M}$ each, then equilibrium concentration of $\mathrm{D}$ (in $\mathrm{mol} \mathrm{L}^{-1}$ ) will be :
Correct Option: , 4
Solution: