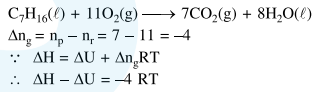Question: The difference between $\Delta \mathrm{H}$ and $\Delta \mathrm{U}(\Delta \mathrm{H}-\Delta \mathrm{U})$, when the combustion of one mole of heptane (1) is carried out at a temperature $T$, is equal to:
3RT
$-3 \mathrm{RT}$
$-4 R T$
4RT
Correct Option: , 3
Solution: