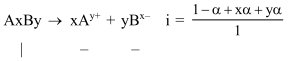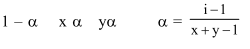Question: The degree of dissociation (1) of a weak electrolyte, $A_{x} B_{y}$ is related to van't Hoff factor (i) by the expression :-
$\alpha=\frac{x+y-1}{i-1}$
$\alpha=\frac{x+y+1}{i-1}$
$\alpha=\frac{i-1}{(x+y-1)}$
$\alpha=\frac{i-1}{x+y+1}$
Correct Option: , 3
Solution: