Question:
The correct statement about $\mathrm{B}_{2} \mathrm{H}_{6}$ is:
Correct Option: 1
Solution:
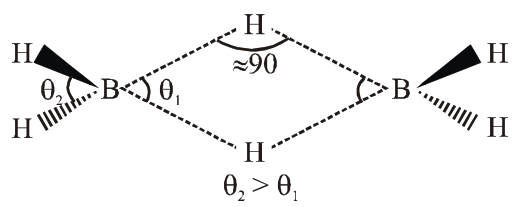
- $\quad \theta_{2}>\theta_{1}, \therefore \mathrm{B}-\mathrm{H}$ (terminal) having less $\mathrm{p}-$ character as compare to bridge bond.
- Both B-H-B bridge bond having same bond length.
- B-H-B bond angle is $\approx 90^{\circ}$
- $\quad \mathrm{BH}_{3}$ is $\mathrm{e}^{-}$deficient species and therefore act as lewis acid
