Question:
The correct order of the atomic radii of $\mathrm{C}, \mathrm{Cs}, \mathrm{Al}$ and $S$ is:
Correct Option: , 4
Solution:
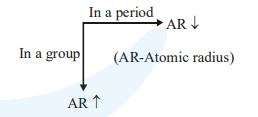
Atomic radii order : $\mathrm{C}<\mathrm{S}<\mathrm{Al}<\mathrm{Cs}$
Atomic radius of $\mathrm{C}: 170 \mathrm{pm}$
Atomic radius of S : $180 \mathrm{pm}$
Atomic radius of $\mathrm{Al}: 184 \mathrm{pm}$
Atomic radius of $\mathrm{Cs}: 300 \mathrm{pm}$
