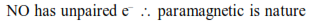Question: The correct order of bond dissociation energy among $\mathrm{N}_{2}, \mathrm{O}_{2}, \mathrm{O}_{2}$-is shown in which of the following arrangements?
$\mathrm{N}_{2}>\mathrm{O}_{2}>\mathrm{O}_{2}^{-}$
$\mathrm{O}_{2}>\mathrm{O}_{2}^{-}>\mathrm{N}_{2}$
$\mathrm{N}_{2}>\mathrm{O}_{2}^{-}>\mathrm{O}_{2}$
$\mathrm{O}_{2}^{-}>\mathrm{O}_{2}>\mathrm{N}_{2}$
Correct Option: 1
Solution: