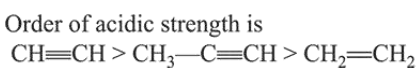Question: The correct order for acid strength of compounds $\mathrm{CH} \equiv \mathrm{CH}$,
$\mathrm{CH}_{3}-\mathrm{C} \equiv \mathrm{CH}$ and $\mathrm{CH}_{2}=\mathrm{CH}_{2}$ is as follows:
$\mathrm{CH} \equiv \mathrm{CH}>\mathrm{CH}_{2}=\mathrm{CH}_{2}>\mathrm{CH}_{3}-\mathrm{C} \equiv \mathrm{CH}$
$\mathrm{CH}_{3}-\mathrm{C} \equiv \mathrm{CH}>\mathrm{CH} \equiv \mathrm{CH}>\mathrm{CH}_{2}=\mathrm{CH}_{2}$
$\mathrm{CH}_{3}-\mathrm{C} \equiv \mathrm{CH}>\mathrm{CH}_{2}=\mathrm{CH}_{2}>\mathrm{HC} \equiv \mathrm{CH}$
$\mathrm{HC} \equiv \mathrm{CH}>\mathrm{CH}_{3}-\mathrm{C} \equiv \mathrm{CH}>\mathrm{CH}_{2}=\mathrm{CH}_{2}$
Correct Option: , 4
Solution: