Question:
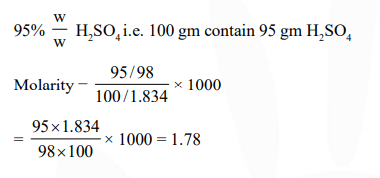
The concentrated sulphuric acid that is peddled commercially is $95 \% \mathrm{H}_{2} \mathrm{SO}_{4}$ by weight. If the density of this commerical acid is $1.834 \mathrm{~g} \mathrm{~cm}^{-3}$, the molarity of this solution is :-
Correct Option: 1
Solution: