Question:
The calculated magnetic moments (spin only value) for species $\left[\mathrm{FeCl}_{4}\right]^{2-},\left[\mathrm{Co}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)_{3}\right]^{3-}$ and
$\mathrm{MnO}_{4}^{2-}$ respectively are :
Correct Option: , 2
Solution:
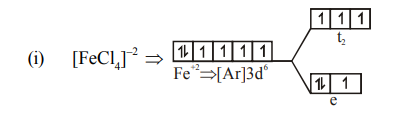
$\mu=\sqrt{n(n+2)} B M$
$=\sqrt{4(4+2)} \mathrm{BM}$
$=\sqrt{24} \mathrm{BM} \Rightarrow 4.90 \mathrm{BM}$
(ii) $\left[\mathrm{Co}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)_{3}\right]^{-3}$
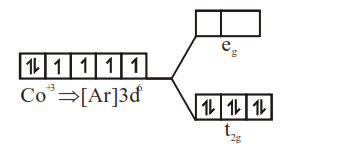
µ = 0
(iii) $\mathrm{MnO}_{4}^{-2}$
$\mathrm{Mn}^{+6} \Rightarrow[\mathrm{Ar}] 3 \mathrm{~d}^{1} \quad \mu=\sqrt{\mathrm{n}(\mathrm{n}+2)} \mathrm{BM}$
$=\sqrt{1(1+2)} \mathrm{BM}$
$=\sqrt{3} \mathrm{BM} \Rightarrow 1.73 \mathrm{BM}$
