Question:
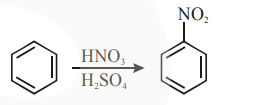
In the above reaction, $3.9 \mathrm{~g}$ of benzene on nitration gives $4.92 \mathrm{~g}$ of nitrobenzene. The percentage yield of nitrobenzene in the above reaction is \%. (Round off to the Nearest Integer).
(Given atomic mass: $\mathrm{C}: 12.0 \mathrm{u}, \mathrm{H}: 1.0 \mathrm{u}$, $\mathrm{O}: 16.0 \mathrm{u}, \mathrm{N}: 14.0 \mathrm{u})$
Solution:
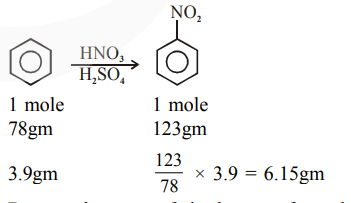
But actual amount of nitrobenzene formed is $4.92 \mathrm{gm}$ and hence.
Percentage yield $=\frac{4.92}{6.15} \times 100=80 \%$
