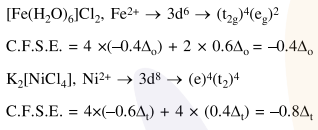Question: The crystal fied stabilization energy (CFSE) of $\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{2}$ and $\mathrm{K}_{2}\left[\mathrm{NiCl}_{4}\right]$, respectively, are :-
$-0.4 \Delta_{\mathrm{O}}$ and $-0.8 \Delta_{\mathrm{t}}$
$-0.4 \Delta_{\mathrm{O}}$ and $-1.2 \Delta_{\mathrm{t}}$
$-2.4 \Delta_{\mathrm{O}}$ and $-1.2 \Delta_{\mathrm{t}}$
$-0.6 \Delta_{\mathrm{O}}$ and $-0.8 \Delta_{\mathrm{t}}$
Correct Option: 1
Solution: