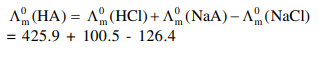Question:
$\wedge_{\mathrm{m}}^{\circ}$ for $\mathrm{NaCl}, \mathrm{HCl}$ and $\mathrm{NaA}$ are $126.4,425.9$ and $100.5 \mathrm{~S} \mathrm{~cm}^{2} \mathrm{~mol}^{-1}$, respectively. If the conductivity of $0.001 \mathrm{M} \mathrm{HA}$ is $5 \times 10^{-5} \mathrm{~S} \mathrm{~cm}^{-1}$, degree of dissociation of $\mathrm{HA}$ is :
Correct Option: , 2
Solution: