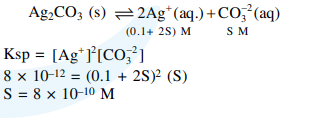Question: If $\mathrm{K}_{\mathrm{sp}}$ of $\mathrm{Ag}_{2} \mathrm{CO}_{3}$ is $8 \times 10^{-12}$, the molar solubility of $\mathrm{Ag}_{2} \mathrm{CO}_{3}$ in $0.1 \mathrm{M} \mathrm{AgNO} 3$ is :
$8 \times 10^{-12} \mathrm{M}$
$8 \times 10^{-10} \mathrm{M}$
$8 \times 10^{-11} \mathrm{M}$
$8 \times 10^{-13} \mathrm{M}$
Correct Option: , 2
Solution: